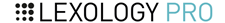Boiling water might mitigate microplastics
A new study suggests that boiling tap water might destroy microplastics in water to make it safer to drink

Concerned About Microplastics in Your Water?
Consider Boiling It First
Boiling tap water traps nano- and microplastic particles inside limescale deposits, a new study has found.
Worried about microplastic particles in your tap water? Just boil it.
At least, that’s the suggestion put forward by researchers at Guangzhou Medical University and Jinan University, China.
In a new study, published in Environmental Science & Technology Letters, the team tested whether boiling your water might have any effect on the tiny nanoplastics and microplastics that are sometimes present in tap water. They found that boiling water effectively traps the plastic particles inside the limescale deposits that build up on a kettle’s inner surfaces.
Could boiling your drinking water reduce exposure to microplastics?
Numerous studies have found evidence of microplastics in real-world tap water samples, but the health effects of ingesting microplastics from drinking water are still unclear. Early studies suggest these microplastics can accumulate in the body and affect the gut microbiome.
Despite the best efforts of water treatment plants, microplastics and nanoplastics remain tricky to remove from water using standard treatment methods. While there are some advanced water treatment technologies that can tackle this problem, they can be prohibitively expensive for less developed areas.
But what if a common household water treatment technique could already be slashing your exposure to microplastics?
“Drinking boiled water, an ancient tradition in some Asian countries, is supposedly beneficial for human health, as boiling can remove some chemicals and most biological substances,” the researchers wrote. “However, it remains unclear whether boiling is effective in removing NMPs [nano- and microplastics] in tap water.”
In their new study, the researchers used fluorescent particles of polystyrene plastic and examined how they behaved as they were heated in different types of water.
Boiling and filtering slashes microparticle levels by 90%
Tap water can either be considered “hard” or “soft”, depending on how rich it is in calcium and magnesium minerals. These minerals are also responsible for the formation of limescale inside kettles, which is why kettles need to be treated with descaler more frequently in areas with hard water.
The researchers found that when microplastic-containing water was brought closer to boiling temperatures, the added polystyrene NMPs began to co-precipitate out of the water alongside the minerals, becoming trapped in the crusty limescale deposits formed.
Boiling was able to remove 84% of the NMPs added to hard water samples containing around 180 milligrams of calcium carbonate (CaCO3). This rose to 90% for very hard water samples, containing around 300 mg/L of the mineral.
The boiling practice was also able to remove up to 25% of the NMPs when done on soft water samples (containing less than 60 mg/L CaCO3), suggesting this simple practice may be more broadly applicable.
“Because the occurrence of NMPs and water quality are uneven globally, the efficacy of boiling water in reducing NMPs may vary from region to region,” wrote the researchers. “Nonetheless, our results have ratified a highly feasible strategy to reduce human NMP exposure and established the foundation for further investigations with a much larger number of samples.”
Reference: Yu Z, Wang JJ, Liu LY, Li Z, Zeng EY. Drinking boiled tap water reduces human intake of nanoplastics and microplastics. Environ Sci Technol Lett. 2024. doi: 10.1021/acs.estlett.4c00081
This article is a rework of a press release issued by the American Chemical Society. Material has been edited for length and content.
Published: February 28, 2024
Meet the Author:
Alexander Beadle
Alexander Beadle is a science writer and editor for Technology Networks. Prior to this, he worked as a freelance science writer. Alexander holds an MChem in materials chemistry from the University of St Andrews, where he won a Chemistry Purdie Scholarship and conducted research into zeolite crystal growth mechanisms and the action of single-molecule transistors.
What is Your Reaction?
 Like
1
Like
1
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0