Smart jab can shrink head and neck cancer tumours within six weeks, trial finds – The Guardian
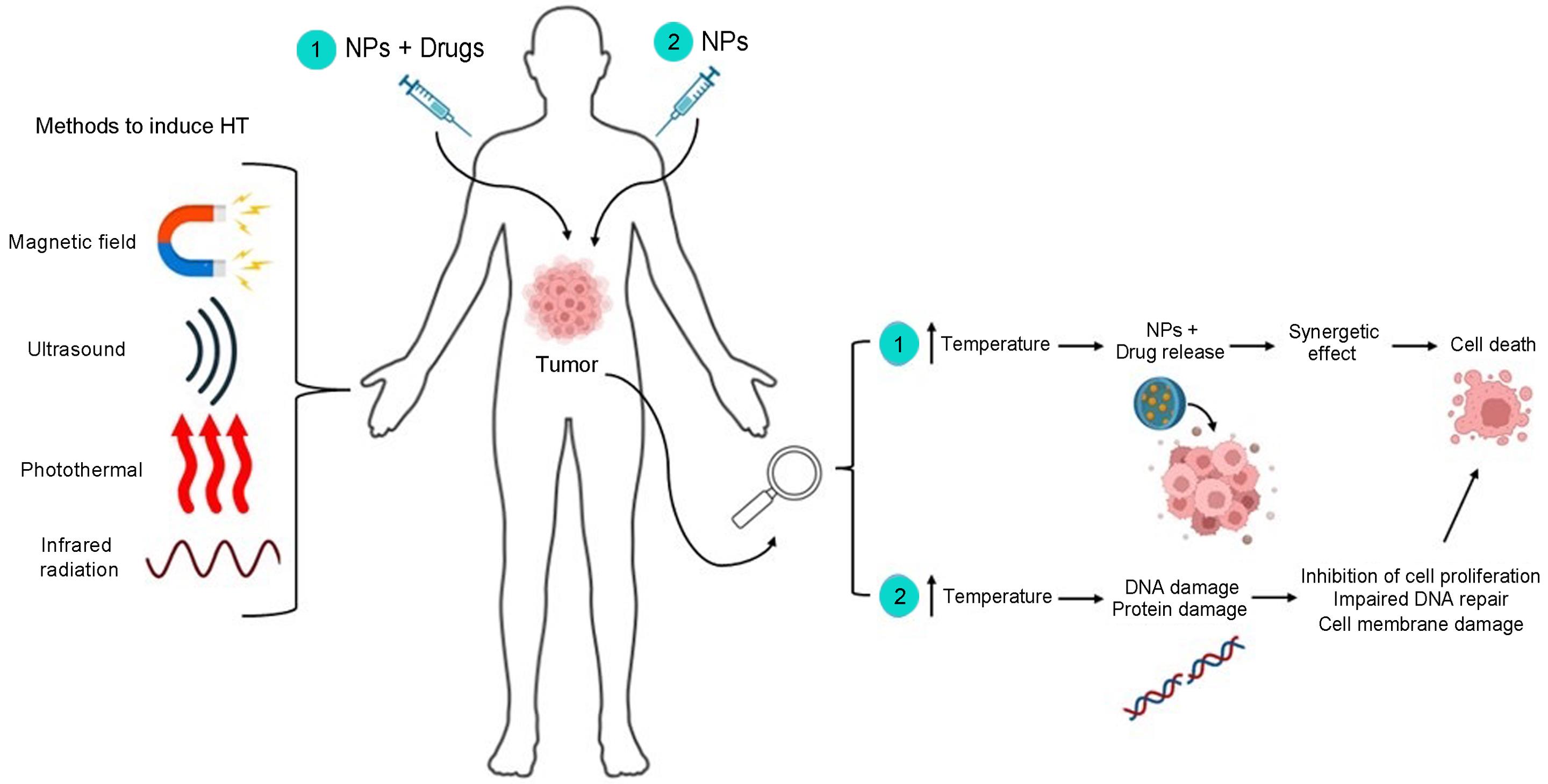
Report on Amivantamab Clinical Trial for Head and Neck Cancer and its Alignment with Sustainable Development Goals
Introduction
A clinical trial has yielded significant results for a new triple-action drug, amivantamab, in treating recurrent or metastatic head and neck cancer. The findings, presented at the European Society for Medical Oncology conference, indicate a substantial advancement in cancer therapy, directly contributing to several United Nations Sustainable Development Goals (SDGs), particularly SDG 3 (Good Health and Well-being).
Clinical Trial Findings: The Orig-AMI 4 Study
Trial Overview
The Orig-AMI 4 trial, funded by Janssen, investigated the efficacy of amivantamab in patients whose head and neck squamous cell carcinoma (HNSCC) had returned or spread after standard treatments.
- Patient Cohort: Individuals with recurrent or metastatic HNSCC who had previously undergone immunotherapy and platinum chemotherapy.
- Intervention: Administration of the drug amivantamab via injection.
- Geographic Scope: The trial included patients from 11 countries, including the United Kingdom.
Key Efficacy Results
Early results from a group of 86 patients demonstrated a high rate of positive outcomes, representing a significant step toward achieving SDG Target 3.4, which aims to reduce premature mortality from non-communicable diseases.
- Tumour Response: 76% of patients experienced tumour shrinkage or a halt in growth.
- Time to Response: Clinical responses were observed within an average of six weeks.
- Progression-Free Survival: The average progression-free survival for patients receiving amivantamab monotherapy was 6.8 months.
- Tolerability: The treatment was generally well tolerated, with most side-effects reported as mild to moderate.
Mechanism of Action and Administration
Triple-Action Therapeutic Approach
Amivantamab is an innovative biologic therapy that targets cancer through three distinct pathways, reflecting the kind of advanced research central to SDG 9 (Industry, Innovation, and Infrastructure).
- It blocks the Epidermal Growth Factor Receptor (EGFR), a protein that facilitates tumour growth.
- It blocks MET, a secondary pathway often used by cancer cells to evade treatment.
- It directs the patient’s own immune system to attack the tumour cells.
Method of Delivery
The drug’s administration method offers notable advantages over conventional intravenous infusions, potentially improving treatment accessibility and aligning with SDG 10 (Reduced Inequalities).
- Administration: Amivantamab is delivered as a simple injection under the skin.
- Benefits: This method is faster and more convenient for patients, reducing time spent in hospital settings.
- Future Potential: The simplicity of administration opens possibilities for delivery in outpatient clinics or, eventually, at home, thereby reducing the burden on healthcare infrastructure and patients.
Alignment with Sustainable Development Goals (SDGs)
SDG 3: Good Health and Well-being
This medical breakthrough directly supports SDG 3 by providing a new, effective treatment for a life-threatening, non-communicable disease. By offering hope to patients with limited options, it improves survival rates and enhances quality of life, as evidenced by patient testimony describing reduced pain and improved function.
SDG 9: Industry, Innovation, and Infrastructure
The development of amivantamab is a prime example of progress under SDG 9. It represents a significant investment in scientific research and innovation (Target 9.5) by the pharmaceutical industry to address a critical health challenge. This “smart drug” technology showcases the potential of advanced biological therapies to transform healthcare.
SDG 10: Reduced Inequalities
The subcutaneous injection delivery model has the potential to advance SDG 10. By simplifying treatment administration, it can make advanced cancer care more accessible to patients who may face geographic or logistical barriers to accessing specialized hospital facilities for lengthy infusions, thereby promoting more equitable health outcomes.
Conclusion
The results of the Orig-AMI 4 trial for amivantamab represent a highly encouraging development in the treatment of head and neck cancer. The drug’s efficacy, combined with its innovative mechanism and convenient delivery method, signals a potential paradigm shift in patient care. This advancement not only offers a new therapeutic option but also strongly aligns with global objectives for health, innovation, and equity as outlined in the Sustainable Development Goals.
Analysis of SDGs, Targets, and Indicators
1. Which SDGs are addressed or connected to the issues highlighted in the article?
-
SDG 3: Good Health and Well-being
- The article’s central theme is a medical advancement in cancer treatment. It discusses a new drug, amivantamab, that has shown positive results in shrinking tumours for head and neck cancer patients, directly contributing to improving health outcomes and well-being for individuals with this non-communicable disease.
-
SDG 9: Industry, Innovation, and Infrastructure
- The development of the “triple-action smart jab” represents a significant scientific and technological innovation in the pharmaceutical industry. The article highlights the research and development process, including a clinical trial (Orig-AMI 4) funded by a pharmaceutical company (Janssen), which falls under the umbrella of enhancing scientific research and innovation.
-
SDG 17: Partnerships for the Goals
- The article describes a multi-stakeholder, international collaboration. The clinical trial was funded by a private company (Janssen) and involved researchers and patients from 11 countries, including public health institutions like the Royal Marsden NHS foundation trust. This public-private, international partnership is crucial for advancing medical research.
2. What specific targets under those SDGs can be identified based on the article’s content?
-
Target 3.4: Reduce premature mortality from non-communicable diseases
- The article directly addresses this target by focusing on a new treatment for head and neck cancer, a major non-communicable disease. The drug amivantamab is designed for patients whose cancer has returned or spread after standard treatments, a group at high risk of premature mortality. The goal of shrinking tumours and stopping their growth is to extend life and improve its quality.
-
Target 9.5: Enhance scientific research and encourage innovation
- The development and testing of amivantamab is a clear example of enhanced scientific research. The article details the innovative “triple-action” mechanism of the drug, which “blocks two key cancer pathways” and “helps the immune system do its job.” The funding of the trial by Janssen and the presentation of results at a major medical conference underscore the commitment to research and development in the health sector.
-
Target 17.17: Encourage and promote effective public, public-private and civil society partnerships
- The Orig-AMI 4 trial is a model of this target. It is a public-private partnership involving the pharmaceutical company Janssen (private sector), the Institute of Cancer Research and the Royal Marsden NHS foundation trust (public/academic sector), and patients across 11 countries. This collaboration pools resources, expertise, and patient access to accelerate medical progress.
3. Are there any indicators mentioned or implied in the article that can be used to measure progress towards the identified targets?
-
Indicator for Target 3.4 (related to mortality and treatment effectiveness)
- The article provides specific data points that can serve as indicators of treatment effectiveness. It states that “76% of this group saw their tumours shrink or stop growing” and that the “average progression-free survival of patients receiving amivantamab on its own was 6.8 months.” These metrics directly measure the impact of the treatment on the disease, which is a proxy for progress towards reducing mortality.
-
Indicator for Target 9.5 (related to R&D and innovation)
- The article implies indicators related to research and development investment. The statement that the trial was “funded by the pharmaceuticals company Janssen” points to private sector R&D expenditure. The development of a novel “triple-action therapy” is a qualitative indicator of successful innovation resulting from this investment.
-
Indicator for Target 17.17 (related to partnerships)
- The article provides a qualitative indicator of a successful multi-stakeholder partnership. The description of the trial involving “patients from 11 countries,” funded by a private company and led by researchers from public institutions, serves as concrete evidence of an effective international, public-private partnership in action.
Summary Table of SDGs, Targets, and Indicators
| SDGs | Targets | Indicators Identified in the Article |
|---|---|---|
| SDG 3: Good Health and Well-being | Target 3.4: By 2030, reduce by one third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being. |
|
| SDG 9: Industry, Innovation, and Infrastructure | Target 9.5: Enhance scientific research, upgrade the technological capabilities of industrial sectors in all countries…encouraging innovation and substantially increasing the number of research and development workers…and public and private research and development spending. |
|
| SDG 17: Partnerships for the Goals | Target 17.17: Encourage and promote effective public, public-private and civil society partnerships, building on the experience and resourcing strategies of partnerships. |
|
Source: theguardian.com

What is Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0





























































/environment-climate-change-and-health-(ech)/water-sanitation-hygiene-and-health-(wsh)/landfill-tuvalu-36092.tmb-1200v.jpg?sfvrsn=5c21fe40_1#)







