Actinium Announces Superior Anti-Tumor Activity of ATNM-400 in Lung Cancer Compared to the Leading First, Second and Third-Line Approved EGFR Mutant Therapies and Mechanistic Synergy with Osimertinib at the AACR-NCI-EORTC International Confer – PR Newswire
Report on Preclinical Data for ATNM-400 in Non-Small Cell Lung Cancer (NSCLC)
Actinium Pharmaceuticals, Inc. has presented preclinical data for ATNM-400, a novel, first-in-class antibody radioconjugate utilizing the alpha-emitter Actinium-225 (Ac-225). The data, presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, focuses on the efficacy of ATNM-400 in Epithelial Growth Factor Receptor (EGFR)-mutant NSCLC models. The findings indicate significant potential for ATNM-400 as both a monotherapy and a combination therapy, aligning with global efforts to advance medical science and improve health outcomes.
Contribution to Sustainable Development Goal 3: Good Health and Well-being
The development and evaluation of ATNM-400 directly support SDG 3, which aims to ensure healthy lives and promote well-being for all at all ages. By targeting a prevalent non-communicable disease like lung cancer, this research contributes to SDG Target 3.4, which seeks to reduce premature mortality from such diseases through advanced treatment.
Advancing Cancer Therapeutics to Improve Patient Outcomes
The preclinical results for ATNM-400 demonstrate a potential breakthrough in treating EGFR-mutant NSCLC, a condition with significant unmet medical needs due to eventual treatment resistance. The study highlights the agent’s capacity to improve upon existing standards of care.
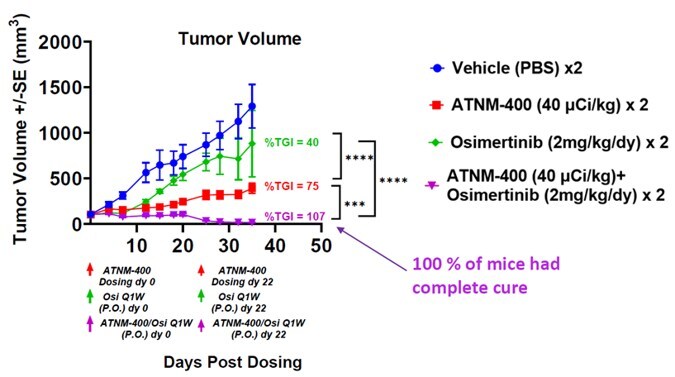
- Superior Monotherapy Efficacy: ATNM-400 exhibited 3-5 times greater tumor growth inhibition compared to established first-line (osimertinib), second-line (Dato-DXd), and third-line (amivantamab) therapies.
- Synergistic Combination Therapy: When combined with osimertinib, ATNM-400 resulted in complete tumor regression in 100% of the animal models studied, suggesting a powerful synergistic effect that could significantly improve therapeutic outcomes.
- Overcoming Treatment Resistance: The ATNM-400 target antigen is linked to treatment resistance. The agent’s efficacy in this context suggests a new strategy to help patients for whom current therapies are no longer effective.
Alignment with Sustainable Development Goal 9: Industry, Innovation, and Infrastructure
This research exemplifies progress toward SDG 9, particularly Target 9.5, which encourages enhancing scientific research and upgrading technological capabilities. The development of ATNM-400 represents a significant innovation in the field of targeted radiotherapy, leveraging advanced scientific principles to create a novel therapeutic candidate.
Technological Innovation in Targeted Radiotherapy
ATNM-400 is a highly innovative therapeutic candidate that combines a monoclonal antibody with the potent alpha-emitting isotope Ac-225. This approach allows for the precise delivery of radiation to cancer cells, inducing irreversible double-strand DNA breaks while potentially minimizing off-target effects.
Mechanistic Rationale and Clinical Potential
The study provides a strong mechanistic rationale for the observed efficacy, supporting its progression into clinical development.
- Target Antigen Expression: The target antigen for ATNM-400 is overexpressed in NSCLC. Critically, treatment with the EGFR inhibitor osimertinib was found to significantly increase the expression of this target antigen.
- Enhanced Cytotoxic Effect: The increased target expression post-osimertinib treatment led to a notable increase in ATNM-400’s cytotoxic effect in vitro, providing a clear mechanism for the observed synergy in combination therapy.
- Clinical Precedent: Existing clinical data showing improved progression-free survival with the combination of osimertinib and external beam radiotherapy provides a strong rationale for combining it with a targeted alpha-therapy like ATNM-400.
Future Implications and Multi-Tumor Potential
Addressing a Global Health Challenge
Lung cancer is the most common cancer worldwide, and NSCLC accounts for approximately 85% of cases. Therapies for EGFR-mutant NSCLC generated approximately $7 billion in sales in 2024, yet resistance remains a major challenge. ATNM-400 is positioned to address this significant unmet need across multiple treatment lines.
Validation of a Multi-Indication Platform
The positive data in NSCLC, coupled with ongoing studies in prostate cancer, validates the multi-tumor potential of ATNM-400. This broad applicability underscores its potential to become a significant therapeutic agent across several major cancer types, contributing to improved health and well-being on a global scale.
Analysis of Sustainable Development Goals (SDGs) in the Article
1. Which SDGs are addressed or connected to the issues highlighted in the article?
-
SDG 3: Good Health and Well-being
- The article’s primary focus is on the development of a new cancer therapy, ATNM-400, for non-small cell lung cancer (NSCLC) and prostate cancer. This directly relates to ensuring healthy lives and promoting well-being by advancing medical treatments for major non-communicable diseases. The text highlights the goal of “improving outcomes for patients” and addressing a “significant unmet need” for those who develop resistance to existing therapies.
-
SDG 9: Industry, Innovation, and Infrastructure
- The article describes Actinium Pharmaceuticals as a “pioneer in the development of differentiated targeted radiotherapies” and details the creation of a “novel, multi-indication first-in-class antibody radioconjugate.” This showcases industrial innovation and advanced scientific research. The company’s efforts to develop new technologies, such as using the alpha-emitter Actinium-225, and holding “approximately 240 patents and patent applications” directly contribute to fostering innovation.
-
SDG 17: Partnerships for the Goals
- The article mentions that Actinium is “engaged with the National Cancer Institute (NCI) under a Cooperative Research and Development Agreement (CRADA).” This is a clear example of a public-private partnership aimed at advancing medical research and development, which is a key aspect of SDG 17.
2. What specific targets under those SDGs can be identified based on the article’s content?
-
Under SDG 3: Good Health and Well-being
- Target 3.4: By 2030, reduce by one third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being. The development of ATNM-400 is a direct effort to improve the *treatment* of cancer (a non-communicable disease), with the article stating its potential to “improve survival outcomes” and overcome resistance to current therapies, thereby contributing to reducing mortality.
- Target 3.b: Support the research and development of vaccines and medicines for the communicable and non-communicable diseases. The entire article is a testament to this target, as it details the “preclinical data” and ongoing “clinical development” of a new medicine for cancer, a major non-communicable disease.
-
Under SDG 9: Industry, Innovation, and Infrastructure
- Target 9.5: Enhance scientific research, upgrade the technological capabilities of industrial sectors in all countries… encouraging innovation and substantially increasing the number of research and development workers… and public and private research and development spending. The article exemplifies this target by describing Actinium’s work as a “truly innovative approach” and a “pioneer” in its field. The presentation of research at the “AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics” is a direct outcome of dedicated R&D spending and scientific research.
-
Under SDG 17: Partnerships for the Goals
- Target 17.17: Encourage and promote effective public, public-private and civil society partnerships. The article explicitly mentions a “Cooperative Research and Development Agreement (CRADA)” with the National Cancer Institute (NCI), a government agency. This collaboration between a private company (Actinium) and a public institution (NCI) to develop cancer treatments is a direct implementation of this target.
3. Are there any indicators mentioned or implied in the article that can be used to measure progress towards the identified targets?
-
For Target 3.4 (Reduce mortality from NCDs):
- Tumor Growth Inhibition (TGI): The article states that “ATNM-400 exhibits superior efficacy with 3-5x greater tumor growth inhibition” compared to other therapies. This is a direct measure of treatment effectiveness.
- Complete Tumor Regression Rate: The finding that the “combination of ATNM-400 and osimertinib resulted in complete tumor regression in 100% of tumor bearing animals” serves as a powerful indicator of therapeutic success.
- Progression-Free Survival (PFS): The article mentions that “improved progression free survival has been demonstrated clinically with the combination of osimertinib and external beam radiotherapy,” providing a rationale for ATNM-400’s use. PFS is a standard clinical endpoint for measuring treatment efficacy.
-
For Target 3.b (Support R&D of new medicines):
- Number of New Drug Candidates in Development: The article presents ATNM-400 as a “lead solid tumor program” and mentions other candidates like “Actimab-A” and “Iomab-ACT,” indicating a pipeline of new medicines under development.
- Presentation of Research Findings: The announcement of “the presentation of the first ever preclinical data of ATNM-400” at a major international conference is an indicator of active and progressing research.
-
For Target 9.5 (Enhance scientific research and innovation):
- Number of Patents and Patent Applications: The statement that “Actinium holds approximately 240 patents and patent applications” is a quantifiable indicator of innovation and intellectual property generation.
- Development of First-in-Class Therapies: ATNM-400 is described as a “first-in-class” therapy, which indicates a high level of innovation that pushes the boundaries of existing technology.
-
For Target 17.17 (Promote partnerships):
- Number and Nature of Public-Private Partnerships: The existence of the “Cooperative Research and Development Agreement (CRADA) for development of Actimab-A” between Actinium and the NCI is a specific, named indicator of a partnership to achieve common goals.
4. Summary Table of SDGs, Targets, and Indicators
| SDGs | Targets | Indicators |
|---|---|---|
| SDG 3: Good Health and Well-being |
3.4: Reduce premature mortality from non-communicable diseases through treatment.
3.b: Support the research and development of medicines for non-communicable diseases. |
|
| SDG 9: Industry, Innovation, and Infrastructure | 9.5: Enhance scientific research and encourage innovation. |
|
| SDG 17: Partnerships for the Goals | 17.17: Encourage and promote effective public-private partnerships. |
|
Source: prnewswire.com
What is Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0















































































